

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Rutherford's experiment indicated that matter was not as uniform as it appears what part of his experimental results implied this idea
Answers: 1

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 03:10
Agas diffuses 1/7 times faster than hydrogen gas (h2). what is the molar mass of the gas? 100.10 g/mol 98.78 g/mol 86.68 g/mol 79.98 g/mol
Answers: 3

You know the right answer?
AB2 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using th...
Questions








Mathematics, 17.07.2019 11:20

Mathematics, 17.07.2019 11:20




Mathematics, 17.07.2019 11:20

Mathematics, 17.07.2019 11:20

History, 17.07.2019 11:20


Chemistry, 17.07.2019 11:20


Mathematics, 17.07.2019 11:20

Mathematics, 17.07.2019 11:20

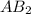 is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places.
is a molecule that reacts readily with water. Calculate the bond energy of the A–B bond using the standard enthalpy of reaction and the bond energy data provided. Enter a number in kJ to 0 decimal places. 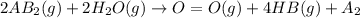
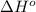 = –142 kJ
= –142 kJ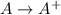
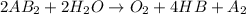
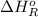 = -142 kJ
= -142 kJ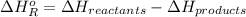
![[(2 \times 2 (A-B) + 2 \times 2 (O-H)] - [(O=O) + 4(H-B) + (A-A)]](/tpl/images/0603/6629/cfc5d.png)
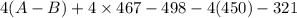
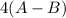 = -142 - 1868 + 498 + 1800 + 321
= -142 - 1868 + 498 + 1800 + 321

