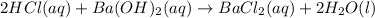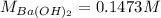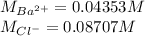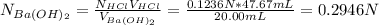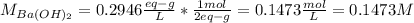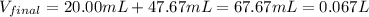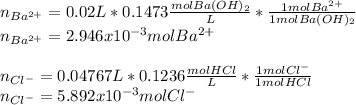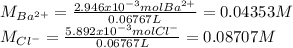A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of a 0.1236 M HCl solution. Write the balanced molecular equation for the neutralization reaction between HCl and Ba(OH)_2 in aqueous solution. Include physical states. 2HCl(aq) + Ba(OH)_2(aq) rightarrow BaCl_2(aq) + 2H_2O(l) Calculate the concentration of Ba(OH)_2 in the original 20.00 mL solution Calculate the concentrations of Ba^2+ and Cl^- in solution following the neutralization reaction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Match each object to its description: a. coma of a comet b. comet tail c. oort cloud haze surrounding a nucleus created by solar wind. hypothetical sphere around the solar system
Answers: 1

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 20:00
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1
You know the right answer?
A 20.00 mL Ba(OH)_2 solution of unknown concentration was neutralized by the addition of 47.67 mL of...
Questions

English, 02.12.2021 01:00

Social Studies, 02.12.2021 01:00

Computers and Technology, 02.12.2021 01:00



Mathematics, 02.12.2021 01:00


Biology, 02.12.2021 01:00











History, 02.12.2021 01:00