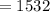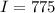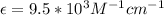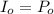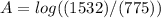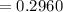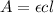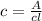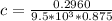Chemistry, 16.04.2020 01:01 Scienceissofun6453
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I ) of 775 when the irradiance of the beam incident to the sample ( P 0 ) was 1532 and the length of the medium ( b ) was 0.875 cm. Anthracene has a molar extinction coefficient ( ϵ ) of 9.5 × 10 3 M − 1 ⋅ cm − 1 . The proportionality constant k ′ for anthracene is 0.30.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 21:00
Need what is special about water as a compound? how does water regulate climate? what drives water evaporation? why is the water vapor fresh water when it rises from the ocean? why might freshwater in the form of snow take longer to enter the water cycle again than liquid precipitation? what is an aquifer? what role do people play in the water cycle? plz just answer as many as you can ! thx if you !
Answers: 1
You know the right answer?
Calculate the concentration of an anthracene solution which produced a fluorescence intensity ( I )...
Questions



History, 20.11.2020 03:30









English, 20.11.2020 03:30








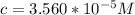
 is
is