Chemistry, 16.04.2020 01:23 cschellfamily
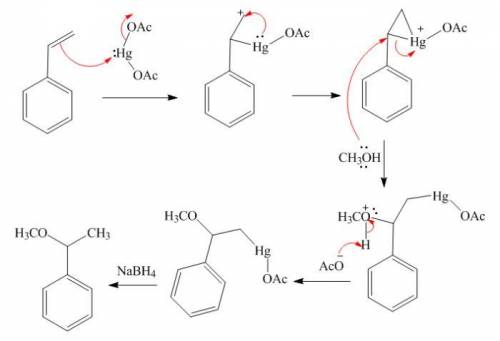
Styrene (1.53 g, 0.011 mol) in methanol (30 mL) was added to a mixture of Hg(OAc)2 (5.30 g, 0.016 mol) in methanol (100 mL) at room temp. and stirred for 24 h. Sodium hydroxide (3.0 M, 16 mL) was added, followed by NaBH4 (0.32 g, 0.008 mol) in NaOH (3.0 M, 16 mL) at 0 °C. The precipitated Hg was removed by filtration. The product was isolated by diethyl ether extraction. After drying over Na2SO4, solvent was removed and distillation gave the product. (Adapted from: Senda, Y.; Kanto, H.; Itoh, H. J. Chem. Soc., Perkin Trans. 2 1997, 1143-1146.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
Styrene (1.53 g, 0.011 mol) in methanol (30 mL) was added to a mixture of Hg(OAc)2 (5.30 g, 0.016 mo...
Questions

Physics, 07.10.2019 23:40

Mathematics, 07.10.2019 23:40



History, 07.10.2019 23:40

Mathematics, 07.10.2019 23:40

History, 07.10.2019 23:40



History, 07.10.2019 23:40

Health, 07.10.2019 23:40

Mathematics, 07.10.2019 23:40


Mathematics, 07.10.2019 23:40



Physics, 07.10.2019 23:50

English, 07.10.2019 23:50