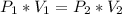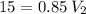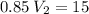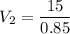I have added 15 L of air to a balloon at sea level (1.0 atm). If I take the
balloon with me to...

Chemistry, 16.04.2020 01:33 battlemarshmell
I have added 15 L of air to a balloon at sea level (1.0 atm). If I take the
balloon with me to Denver, where the air pressure is 0.85 atm, what will
the new volume of the balloon be?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 21.06.2019 20:50
Which real-world scenarios below represent physical and chemical changes? -running a car -exploding fireworks -mixing water and powdered drink mix -combining oil and vinegar to make salad dressing -taking aspirin for a headache -diluting bleach with water-digesting dinner-spreading peanut butter on bread
Answers: 2


Chemistry, 22.06.2019 21:30
If 22.5 of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. what is the new volume?
Answers: 1
You know the right answer?
Questions

Social Studies, 13.03.2022 02:20

Chemistry, 13.03.2022 02:20







Physics, 13.03.2022 02:30




Mathematics, 13.03.2022 02:30


Mathematics, 13.03.2022 02:30




Mathematics, 13.03.2022 02:30