
The standard enthalpy change for the combustion of 1 mole of propane is –2043.0 kJ. C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g) Calculate ΔfH° for propane based on the following standard molar enthalpies of formation. molecule ΔfH° (kJ/mol-rxn) CO2(g) –393.5 H2O(g) –241.8'

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Select all that apply. a beta particle: is electromagnetic energy is an electron has zero charge is emitted from the nucleus has a +2 charge has a -1 charge
Answers: 1

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1
You know the right answer?
The standard enthalpy change for the combustion of 1 mole of propane is –2043.0 kJ. C3H8(g) + 5 O2(g...
Questions

Chemistry, 06.10.2020 14:01

Chemistry, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01



Mathematics, 06.10.2020 14:01




Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01


Mathematics, 06.10.2020 14:01



History, 06.10.2020 14:01


History, 06.10.2020 14:01

Mathematics, 06.10.2020 14:01

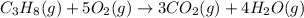
![\Delta H^o_{rxn}=[(3\times \Delta H^o_f_{(CO_2(g))})+(4\times \Delta H^o_f_{(H_2O(g))})]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times \Delta H^o_f_{(O_2(g))})]](/tpl/images/0604/1723/6eb42.png)
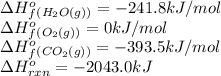
![-2043.0=[(3\times (-393.5))+(4\times (-241.8))]-[(1\times \Delta H^o_f_{(C_3H_8(g))})+(5\times (0))]\\\\\Delta H^o_f_{(C_3H_8(g))}=-104.7kJ/mol](/tpl/images/0604/1723/1b82f.png)
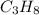 is -104.7 kJ/mol
is -104.7 kJ/mol

