Chemistry, 16.04.2020 02:07 EmmaPreston43
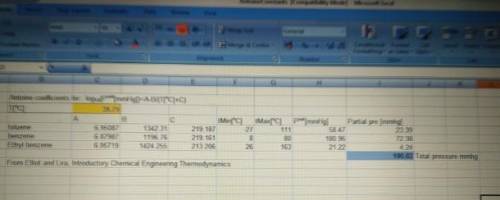
The production of ethylbenzene, a very popular industrial chemical, is carried out reacting benzene with ethylene in liquid phase. This reaction takes place in a series of reactors that involve multiple side reactions and intermediates. Ethylene, being the limiting reactant, is used up first and hence a considerable amount of benzene remains unreacted. From one of the reactors in the series, the exit stream is a mixture of this unreacted benzene (1), an intermediate – toluene (2), and the product ethyl benzene (3).
It is desirable to separate this liquid mixture before sending the components to the next series of reactors/process steps. So 100 mol/min of this mixture is flashed from 200 mm Hg and 50 °C to 100 mm Hg. If the mole fraction of benzene and toluene are 40% each when the mixture enters the flash distillation unit, determine if the mixture will flash completely, partially, or not at all. Assume ideal gas and ideal solution behavior for the vapor phase and liquid phase, respectively. If the mixture does flash partially, determine the composition and molar flow rates of the equilibrium streams exiting the reactor. Show all calculations by hand using your preferred method for solving simultaneous equations. Alternatively, you may use Solver (Excel) but this must be accompanied by a printout of a neatly formatted Excel sheet showing your equations and constraints.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
The production of ethylbenzene, a very popular industrial chemical, is carried out reacting benzene...
Questions

Mathematics, 08.12.2020 19:30

Mathematics, 08.12.2020 19:30


Mathematics, 08.12.2020 19:30

Mathematics, 08.12.2020 19:30



Mathematics, 08.12.2020 19:30




Biology, 08.12.2020 19:30


Mathematics, 08.12.2020 19:30

Biology, 08.12.2020 19:30


Chemistry, 08.12.2020 19:30

History, 08.12.2020 19:30

Mathematics, 08.12.2020 19:30

Biology, 08.12.2020 19:30