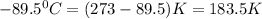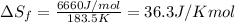Chemistry, 16.04.2020 02:53 tylijahking
Calculate the change in entropy that occurs in the system when 1.24 mol of isopropyl alcohol (c3h8o) melts at its melting point (-89.5 ∘c). heat of fusion is 5.37 kj/mol.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:20
Sodium nitrite (nano2) reacted with 2−iodooctane to give a mixture of two constitutionally isomeric compounds of molecular formula c8h17no2 in a combined yield of 88%. draw reasonable structures for these two isomers. click the "draw structure" button to launch the drawing utility. place the two compounds in the appropriate boxes below.
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
Calculate the change in entropy that occurs in the system when 1.24 mol of isopropyl alcohol (c3h8o)...
Questions

Mathematics, 08.10.2019 01:10



















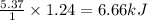

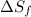 = Entropy of fusion = ?
= Entropy of fusion = ?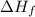 = enthalpy of fusion = 6.66 kJ/mol = 6660 J/mol (1kJ=1000J)
= enthalpy of fusion = 6.66 kJ/mol = 6660 J/mol (1kJ=1000J)