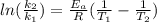The rate constant for a first order reaction is 0.060s-1. when the temperature is increased from 298K to 331 K , the rate constant increases to 0.18s-1. Calculate the activation energy for this reaction. Use kJ/mol for the units. The activation energy in kJ/mol equals (include the units in your answer):

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Seltzer water is created by placing water under pressure with carbon dioxide gas. which of the following statements best describe seltzer water: a. the solution will be slightly acidic b. the solution will be slightly basic. the solution will be strongly acidic. d. the solution will be strongly basic. e. the solution will be neutral
Answers: 3

Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3
You know the right answer?
The rate constant for a first order reaction is 0.060s-1. when the temperature is increased from 298...
Questions


Biology, 24.06.2019 12:00

English, 24.06.2019 12:00


History, 24.06.2019 12:00

History, 24.06.2019 12:00

Mathematics, 24.06.2019 12:00

Mathematics, 24.06.2019 12:00


History, 24.06.2019 12:00






History, 24.06.2019 12:00


Chemistry, 24.06.2019 12:00


Mathematics, 24.06.2019 12:00