Chemistry, 16.04.2020 03:45 skylar1315
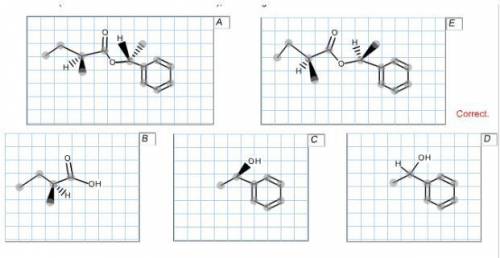
Exactly 2.00 g of an ester A containing only C, H, and O was saponified with 15.00 mL of a 1.00 M NaOH solution. Following the saponification, the solution required 5.30 mL of 1.00 M HCl to titrate the unused NaOH. Ester A, as well as its acid and alcohol saponification products B and C, respectively, were all optically active. Compound A was not oxidized by K2Cr2O7, nor did compound A decolorize Br2 in CH2Cl2. Alcohol C was oxidized to acetophenone by K2Cr2O7. When acetophenone was reduced with NaBH4, a compound D was formed that reacted with the acid chloride derived from B to give two optically active compounds: A (identical to the starting ester) and E. Propose a neutral structure for each compound that is consistent with the data. Note that the absolute stereochemical configurations of chiral substances cannot be determined from the data. Arbitrarily draw a configuration for an enantiomer (and be consistent with the derivatives); omit wedge/dash bonds for a racemate.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1


Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
Exactly 2.00 g of an ester A containing only C, H, and O was saponified with 15.00 mL of a 1.00 M Na...
Questions




English, 18.08.2021 17:30

Mathematics, 18.08.2021 17:30


English, 18.08.2021 17:30


English, 18.08.2021 17:30


English, 18.08.2021 17:30

Computers and Technology, 18.08.2021 17:30

History, 18.08.2021 17:30

Mathematics, 18.08.2021 17:30

Mathematics, 18.08.2021 17:30





Mathematics, 18.08.2021 17:30