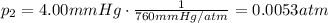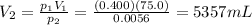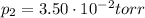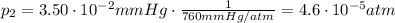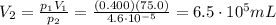Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Describe the chemical reaction based on the chemical equation below. also, explain whether the equation is balanced.
Answers: 1

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 22.06.2019 04:30
Suppose that during that icy hot lab 65,000 j of energy were transferred to 450 g of water at 20°c what would have have been the final temperature of the water
Answers: 2

You know the right answer?
3. A sample of krypton gas occupies 75.0 mL at 0.400 atm. If the temperature remained constant, what...
Questions





Computers and Technology, 13.09.2019 01:20










Computers and Technology, 13.09.2019 01:20


Computers and Technology, 13.09.2019 01:20


Biology, 13.09.2019 01:20

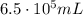
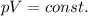
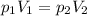
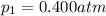 is the initial pressure
is the initial pressure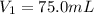 is the initial volume
is the initial volume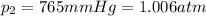 is the final pressure (using the conversion factor
is the final pressure (using the conversion factor 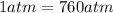 )
)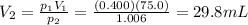
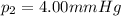 is the final pressure
is the final pressure