Chemistry, 16.04.2020 04:57 pleasedontspamme
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and 10.0 mL of 0.074 M NaOH . 0.074 M NaOH. Next, you add 1.00 mL of 3.51 × 10 − 5 M 3.51×10−5 M lidocaine to this mixture. Denoting lidocaine as L, calculate the fraction of lidocaine present in the form LH +

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3
You know the right answer?
You prepare a buffer solution from 10.0 mL of 0.100 M MOPS (3‑morpholinopropane‑1‑sulfonic acid) and...
Questions

English, 12.03.2021 16:10


History, 12.03.2021 16:10


English, 12.03.2021 16:10

English, 12.03.2021 16:10


English, 12.03.2021 16:10



Mathematics, 12.03.2021 16:10

Mathematics, 12.03.2021 16:10

Mathematics, 12.03.2021 16:10




Mathematics, 12.03.2021 16:10


Chemistry, 12.03.2021 16:10

Mathematics, 12.03.2021 16:10

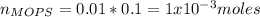
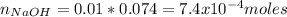
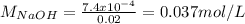
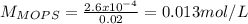
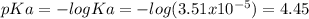
![pH=pKa+log\frac{[NaOH]}{[MOPS]} =4.45+log\frac{0.037}{0.013} =4.9](/tpl/images/0604/7273/c3441.png)
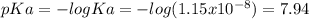
![pH=pKa+log\frac{[base]}{[acid]} \\4.9=7.94+log\frac{[base]}{[acid]}\\log\frac{[base]}{[acid]}=-3.04\\base/acid=9.12x10^{-4}](/tpl/images/0604/7273/6eb33.png)