

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Determine the total pressure of gas mixture the contains oxygen, nitrogen, and helium if the partial...
Questions


English, 26.03.2021 07:20

Mathematics, 26.03.2021 07:20

Mathematics, 26.03.2021 07:20

Advanced Placement (AP), 26.03.2021 07:20

Mathematics, 26.03.2021 07:20

Mathematics, 26.03.2021 07:20



Mathematics, 26.03.2021 07:20

History, 26.03.2021 07:20




Physics, 26.03.2021 07:20

Mathematics, 26.03.2021 07:20

English, 26.03.2021 07:20



Business, 26.03.2021 07:20

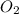 = 0.197 atm
= 0.197 atm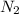 = 0.461 atm
= 0.461 atm

