
Chemistry, 16.04.2020 20:01 Jerryholloway5871
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
2NaN3(s)-> 2Na(s) + 3N2(s)
An unknown amount of sodium azide (NaN3) reacts and produces 744 mL of nitrogen gas. What mass of sodium metal is produced?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Drag each number to the correct location on the equation. each number can be used more than once, but not all numbers will be used. balance the equation with the coefficients. 2 3 4 5 kclo3 -> kcl + o2
Answers: 1

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 21.06.2019 23:00
Plz choose one of the compounds from the table and explain how you know the numbers of atoms in your formula. is it possible for two different compounds to be made from the exact same two elements? why or why not? with a limited number of elements (less than 120 are known), does this mean we also have a small number of compounds or do we have a large number of compounds in this world?
Answers: 1

You know the right answer?
The reaction below shows the decomposition of sodium azide into sodium metal and nitrogen gas.
...
...
Questions



Mathematics, 18.02.2021 01:00

Mathematics, 18.02.2021 01:00


Mathematics, 18.02.2021 01:00

Mathematics, 18.02.2021 01:00

Mathematics, 18.02.2021 01:00

Mathematics, 18.02.2021 01:00


Medicine, 18.02.2021 01:00



Mathematics, 18.02.2021 01:00

Spanish, 18.02.2021 01:00


Mathematics, 18.02.2021 01:00


Mathematics, 18.02.2021 01:00

English, 18.02.2021 01:00

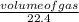
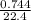 = 0.03moles
= 0.03moles 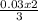 = 0.02mole
= 0.02mole

