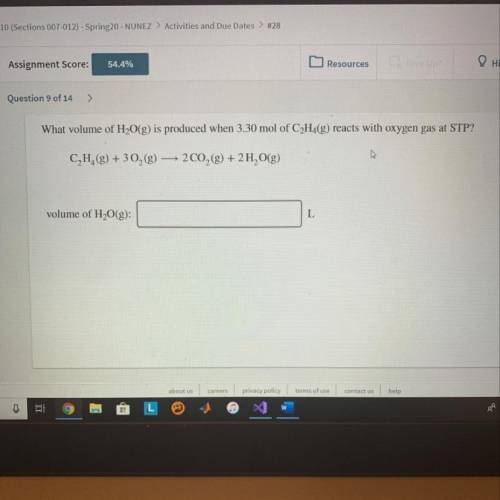
What volume of H2O(g) is produced when 3.30mol of C2H2(g) reacts with oxygen gas at STP?
...

Chemistry, 17.04.2020 05:06 shaunarothh1276
What volume of H2O(g) is produced when 3.30mol of C2H2(g) reacts with oxygen gas at STP?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 07:20
After watching the video "zinc strip in copper nitrate solution", and reading the instructions, click on the link labeled "start" just below the drawing of the pencil tip. follow the direction to complete the 3x3 grid. answer the below questions for the portion of the activity in which sn(s) is placed in agno3(aq)
Answers: 1

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3
You know the right answer?
Questions

Mathematics, 29.08.2021 20:50

Mathematics, 29.08.2021 20:50



English, 29.08.2021 20:50

History, 29.08.2021 20:50

History, 29.08.2021 20:50

Biology, 29.08.2021 20:50

Social Studies, 29.08.2021 20:50


Mathematics, 29.08.2021 20:50



Geography, 29.08.2021 20:50




Mathematics, 29.08.2021 20:50

English, 29.08.2021 20:50



