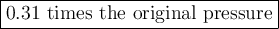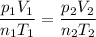Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2
You know the right answer?
If the temperature of the helium balloon were increased from 30°C to 35°C and the volume of the ball...
Questions





Social Studies, 16.03.2022 09:30



Mathematics, 16.03.2022 09:30



Chemistry, 16.03.2022 09:30

Arts, 16.03.2022 09:30


Social Studies, 16.03.2022 09:30

SAT, 16.03.2022 09:30

Computers and Technology, 16.03.2022 09:40




Mathematics, 16.03.2022 09:40