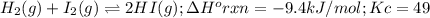H2(g), I2(g), and HI(g) are at equilibrium at a different temperature in a different vessel. (c) When the temperature in the vessel is decreased, does the equilibrium shift to the right, favoring the product, or to the left, favoring the reactants? Justify your answer. Rigid vessel

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
H2(g), I2(g), and HI(g) are at equilibrium at a different temperature in a different vessel. (c) Whe...
Questions

Computers and Technology, 24.09.2019 01:30


Mathematics, 24.09.2019 01:30



Advanced Placement (AP), 24.09.2019 01:30



Mathematics, 24.09.2019 01:30



Mathematics, 24.09.2019 01:30

Chemistry, 24.09.2019 01:30



Chemistry, 24.09.2019 01:30




Mathematics, 24.09.2019 01:30