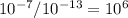Chemistry, 18.04.2020 19:27 martdrea13
A pH scale is shown, fading from red at the left end to blue at the right. Above the scale, pOH is shown in even increments from 14 to zero, and below the scale, p H is shown in even increments from 0 to 14. Points marked are: A at 1 on the p H scale, B at 7 on the p O H scale, C at 13 on the p H scale, and D at 6 on the p H scale.
Based on log rules and the way pH is calculated, what is the difference in [OH– ] concentration between point A and point B.
101
105
106
107

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Astudent carefully transfers 30 g of water and 30 g of alcohol in a glass tube, forming two layers and filling the tube completely. after sealing the tube, the student mixes the solutions, and notices a bubble that forms in the tube. what is the mass of the contents in the glass tube after mixing?
Answers: 2


Chemistry, 22.06.2019 22:30
Which of these statements best explains why space exploration should be encouraged? it prepares humans to live without oxygen. it dispel myths about objects in space. it prevents comets and asteroids from striking earth. it creates technology to absorb harmful radiations in space.
Answers: 1

Chemistry, 23.06.2019 19:30
Given ch4+2o2—> co2+2h2o what mass of oxygen would be needed to form 17 grams of carbon dioxide
Answers: 1
You know the right answer?
A pH scale is shown, fading from red at the left end to blue at the right. Above the scale, pOH is s...
Questions

History, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00


Mathematics, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00






History, 22.12.2020 01:00

Mathematics, 22.12.2020 01:00







Mathematics, 22.12.2020 01:00

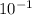 M.
M. 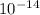 , we can solve for the concentration of OH-:
, we can solve for the concentration of OH-: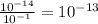
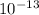 .
.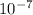 M.
M.