Use the following data to calculate the standard enthalpy of formation of pentane, C5H12(l)?
C...


Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:50
Suppose you got a low yield of benzoin from your benzoin condensation reaction and thus only have 0.300 g of benzoin to use as the starting material for this reaction. how much concentrated nitric acid should you add? (concentrated nitric acid is 15.8 m). write your answer in the form x.xx ml
Answers: 1

Chemistry, 23.06.2019 01:30
Adirect relationship can be represented by: a curve a pie chart
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

You know the right answer?
Questions

Mathematics, 28.01.2020 01:31




History, 28.01.2020 01:31

History, 28.01.2020 01:31


Mathematics, 28.01.2020 01:31

History, 28.01.2020 01:31

History, 28.01.2020 01:31


History, 28.01.2020 01:31


Social Studies, 28.01.2020 01:31

Mathematics, 28.01.2020 01:31

Mathematics, 28.01.2020 01:31



Chemistry, 28.01.2020 01:31


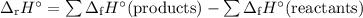
![\begin{array}{rcl}-3535.9 & = & [5\times(-393.5) -6 \times(-285.8)] - x\\-3535.9 & = & -3682.3 - x\\x& = & \textbf{-146.4 kJ/mol}\\\end{array}\\\text{The enthalpy of formation of pentane is } \large \boxed{\textbf{-146.4 kJ/mol}}](/tpl/images/0610/4927/daae3.png)


