
Chemistry, 18.04.2020 22:16 LoveHalo4758
A 5.45 L sample at 10.0°C and a pressure of 64.7 kPa is allowed to expand to a volume of 18.50L. The final pressure of the gas is 58.0 kPa. What is the final temperature of the gas in degrees Celsius?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2
You know the right answer?
A 5.45 L sample at 10.0°C and a pressure of 64.7 kPa is allowed to expand to a volume of 18.50L. The...
Questions

Chemistry, 26.08.2019 20:00


Mathematics, 26.08.2019 20:00


Biology, 26.08.2019 20:00


History, 26.08.2019 20:00




Social Studies, 26.08.2019 20:00


Chemistry, 26.08.2019 20:00

Biology, 26.08.2019 20:00

English, 26.08.2019 20:00





English, 26.08.2019 20:00



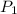 = initial pressure of gas = 64.7 kPa
= initial pressure of gas = 64.7 kPa = final pressure of gas = 50.8 kPa
= final pressure of gas = 50.8 kPa = initial volume of gas = 5.45 L
= initial volume of gas = 5.45 L = final volume of gas = 18.50 L
= final volume of gas = 18.50 L = initial temperature of gas =
= initial temperature of gas = 
 = final temperature of gas = ?
= final temperature of gas = ?



