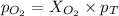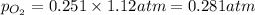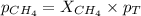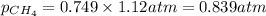Chemistry, 19.04.2020 00:48 st23pgardner
A mixture of 6.00 g of O2 (g) and 9.00 g of CH4 (g) is placed in a 15.0 L vessel at 0o C. What is the partial pressure of each gas? What is the total pressure in the vessel?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 23:00
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1

Chemistry, 23.06.2019 00:00
#7 how does the structure of amino acids allow them to form a polypeptide? each amino acid has an amino group and a carboxyl group. each amino acid has a hydrogen atom and a carboxyl group. each amino acid has a carboxyl group and an r group. each amino acid has an r group and a hydrogen atom.
Answers: 1

You know the right answer?
A mixture of 6.00 g of O2 (g) and 9.00 g of CH4 (g) is placed in a 15.0 L vessel at 0o C. What is th...
Questions


English, 18.10.2019 03:30

Mathematics, 18.10.2019 03:30



Mathematics, 18.10.2019 03:30

History, 18.10.2019 03:30

English, 18.10.2019 03:30


Mathematics, 18.10.2019 03:30



Mathematics, 18.10.2019 03:30







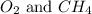 is, 0.281 atm and 0.839 atm respectively.
is, 0.281 atm and 0.839 atm respectively.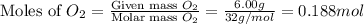
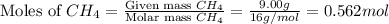
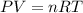
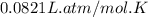
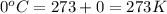
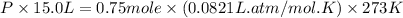
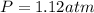
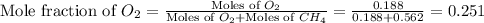
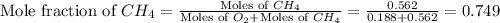
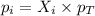
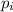 = partial pressure of gas
= partial pressure of gas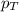 = total pressure of gas = 1.12 atm
= total pressure of gas = 1.12 atm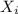 = mole fraction of gas
= mole fraction of gas