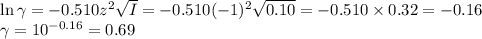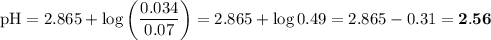We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClCH2COONa) in 1.0 L of water (pKa = 2,865).
to. Calculate the pH
yes. Calculate the pH using the formal forms (activities). Have on
counts the contribution of the protons (section a) in the calculation of the ionic strength.
C. Find the pH of a mixture prepared by dissolving the following compounds
in a final volume of 1L: 0.08 moles of ClCH2COOH, 0.04 moles of
ClCH2COONa, 0.05 moles of HNO3 and 0.06 moles of NaOH

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
An exothermic reaction is conducted in an insulated calorimeter filled with water. the calorimeter is then sealed so that there is no heat exchanged between the contents of the container and the surrounding air. which of the following statements is true about the reaction?
Answers: 1

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3

Chemistry, 22.06.2019 12:50
What is the chemical name of the compound na2co3? use the list of polyatomic ions and the periodic table to you answer. a. sodium carbon oxide b. sodium carbonate c. sodium(ll) carbonate d. sodium oxalate
Answers: 1

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1
You know the right answer?
We mix 0.08 moles of chloroacetic acid (ClCH2COOH) and 0.04 moles of
sodium chloroacetate (ClC...
sodium chloroacetate (ClC...
Questions


History, 04.02.2020 16:51



Mathematics, 04.02.2020 16:51




Spanish, 04.02.2020 16:51

Mathematics, 04.02.2020 16:51

Mathematics, 04.02.2020 16:51



English, 04.02.2020 16:51




Mathematics, 04.02.2020 16:51


![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{[\text{A}^{-}]}{\text{[HA]}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.04}{0.08}\right )\\\\& = & 2.865 + \log0.50 \\& = &2.865 - 0.30 \\& = & \mathbf{2.56}\\\end{array}](/tpl/images/0610/7910/ebc2c.png)
![\text{[H$^{+}$]} = 10^{-\text{pH}} \text{ mol/L} = 10^{-2.56}\text{ mol/L} = 2.73 \times 10^{-3}\text{ mol/L}](/tpl/images/0610/7910/adeed.png)
![I = \dfrac{1}{2} \sum_{i} {c_{i}z_{i}^{2}}\\\\I = \dfrac{1}{2}\left [0.04\times (+1)^{2} + 0.04\times(-1)^{2} + 0.00273\times(+1)^{2}\right]\\\\= \dfrac{1}{2} (0.04 + 0.04 + 0.00273) = \dfrac{1}{2} \times 0.08273 = 0.041](/tpl/images/0610/7910/9dfcb.png)
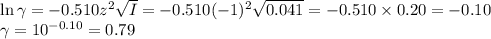
![\begin{array}{rcl}\text{pH} & = & \text{pK}_{\text{a}} + \log \left(\dfrac{a_{\text{A}^{-}}}{a_{\text{[HA]}}}\right )\\\\& = & 2.865 +\log \left(\dfrac{0.032}{0.08}\right )\\\\& = & 2.865 + \log0.40 \\& = & 2.865 -0.40\\& = & \mathbf{2.46}\\\end{array}\\](/tpl/images/0610/7910/7d363.png)
![I = \dfrac{1}{2}\left [0.10\times (+1)^{2} + 0.05 \times(-1)^{2} + 0.05\times(-1)^{2}\right]\\\\= \dfrac{1}{2} (0.10 + 0.05 + 0.05) = \dfrac{1}{2} \times 0.20 = 0.10](/tpl/images/0610/7910/85fb3.png)