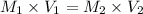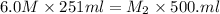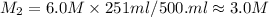Chemistry, 19.04.2020 21:02 hePandaKing6909
If 251 mL of 6.0 M H2SO4 is diluted to 500. mL, what is its new molarity?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1
You know the right answer?
If 251 mL of 6.0 M H2SO4 is diluted to 500. mL, what is its new molarity?...
Questions





Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20

Advanced Placement (AP), 18.03.2021 02:20

Business, 18.03.2021 02:20

Mathematics, 18.03.2021 02:20


Spanish, 18.03.2021 02:20


English, 18.03.2021 02:20


Social Studies, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20


Mathematics, 18.03.2021 02:20