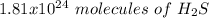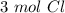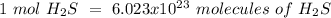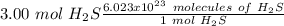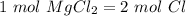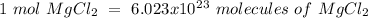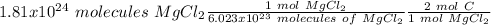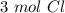Chemistry, 14.11.2019 10:31 MadisonUpky9652
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express your answer numerically in molecules.. calculate the number of moles of cl atoms in 1.81×10^24 formula units of magnesium chloride, mgcl2 .

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3


Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2
You know the right answer?
Using avogadro's number (6.02*10^23): calculate the number of molecules in 3.00 moles h2s . express...
Questions





Mathematics, 08.03.2021 04:50

Social Studies, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50



English, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50

English, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50


History, 08.03.2021 04:50


English, 08.03.2021 04:50

Mathematics, 08.03.2021 04:50