
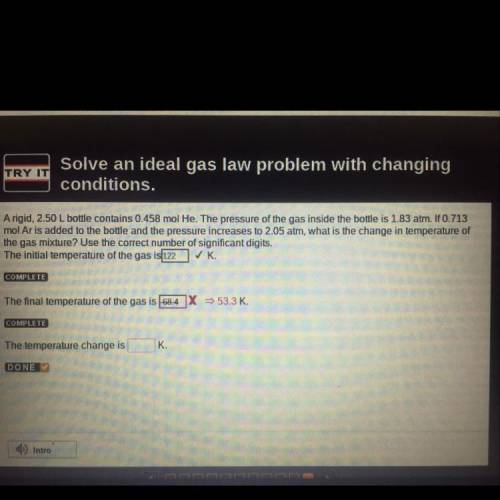
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm. If 0.713
mol Ar is added to the bottle and the pressure increases to 2.05 atm, what is the change in temperature of
the gas mixture? Use the correct number of significant digits.
The temperature change is

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1
You know the right answer?
A rigid, 2.50 L bottle contains 0.458 mol He. The pressure of the gas inside the bottle is 1.83 atm....
Questions




Mathematics, 21.04.2020 19:31

Computers and Technology, 21.04.2020 19:31





Chemistry, 21.04.2020 19:31


Computers and Technology, 21.04.2020 19:31


Mathematics, 21.04.2020 19:31

History, 21.04.2020 19:31

Mathematics, 21.04.2020 19:31



Mathematics, 21.04.2020 19:32




