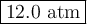Chemistry, 20.04.2020 05:01 samantamartinez
A steel container with a volume of 30L is filled with oxygen to a pressure of 9.00 atm at 28.0°C. What is the pressure of the temperature changes to 129.0°C

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

You know the right answer?
A steel container with a volume of 30L is filled with oxygen to a pressure of 9.00 atm at 28.0°C. Wh...
Questions



History, 10.12.2020 18:30

Spanish, 10.12.2020 18:30



Mathematics, 10.12.2020 18:30


Mathematics, 10.12.2020 18:30

Mathematics, 10.12.2020 18:30

Chemistry, 10.12.2020 18:30


Physics, 10.12.2020 18:30

Mathematics, 10.12.2020 18:30


Mathematics, 10.12.2020 18:30


Mathematics, 10.12.2020 18:30

Mathematics, 10.12.2020 18:30

Mathematics, 10.12.2020 18:30