
Chemistry, 20.04.2020 16:55 zeesharpe05
How many liters of hydrogen gas will be produced at STP from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (H3PO4)
The equation is 3Mg + 2H3(PO4)-->Mg(PO4)2+3H2

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have weak covalent bonds. which of the following is most likely a property of this substance? a. high ph b. high conductivity c. low melting point d. low flammability
Answers: 3
You know the right answer?
How many liters of hydrogen gas will be produced at STP from the reaction of 7.179×10^23 atoms of ma...
Questions

Mathematics, 22.02.2021 03:40

Chemistry, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40



Mathematics, 22.02.2021 03:40

English, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

Chemistry, 22.02.2021 03:40


Social Studies, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40

English, 22.02.2021 03:40

Mathematics, 22.02.2021 03:40


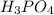 = 54.219 g
= 54.219 g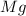 =
= 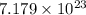
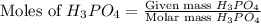
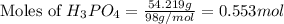
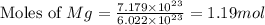
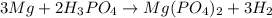
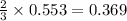 moles of
moles of 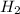
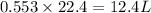 volume of hydrogen gas
volume of hydrogen gas


