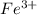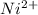Between Lab Period 1 and Lab Period 2, design a separation scheme for all 4 cations. Use the results of your preliminary tests and the reasoning illustrated in the introduction and the Week 2 Worksheet. Be sure to include equations for all the reactions which occur at each step in the scheme. Record the scheme in the data section of the lab notebook. The scheme MUST be submitted at the start of Lab Period 2. It will be reviewed by the TA at the start of the lab period, before you begin testing your scheme. Ag+, Fe 3+ Cu2+, Ni2+

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3

You know the right answer?
Between Lab Period 1 and Lab Period 2, design a separation scheme for all 4 cations. Use the results...
Questions



Physics, 19.06.2020 15:57




Mathematics, 19.06.2020 15:57


Mathematics, 19.06.2020 15:57









Mathematics, 19.06.2020 15:57


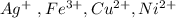
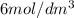 of
of 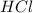 to the mixture solution
to the mixture solution 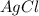 to be formed
to be formed 
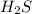 to the remaining
to the remaining 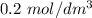 of HCl
of HCl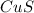 to be formed
to be formed
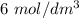 of aqueous
of aqueous 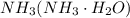 , process the solution in a centrifuge,when the
, process the solution in a centrifuge,when the 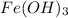 and the remaining solution
and the remaining solution 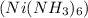
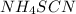 this will
this will 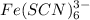 which will have the color of
which will have the color of