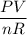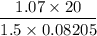At what temperature will 41.6 grams N, exert a pressure of 815 mmHg in a
20.0L cylinder?
...

Chemistry, 20.04.2020 20:02 twistedhyperboles
At what temperature will 41.6 grams N, exert a pressure of 815 mmHg in a
20.0L cylinder?
Hint: You must convert 41.6 g into moles FIRST, then use Ideal gas law to solve
for temperature.
134K
O 238 K
O 337 K.
O 176K

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
Questions




Advanced Placement (AP), 14.01.2021 06:30


Mathematics, 14.01.2021 06:30





Mathematics, 14.01.2021 06:40

Mathematics, 14.01.2021 06:40

Mathematics, 14.01.2021 06:40

History, 14.01.2021 06:40

Spanish, 14.01.2021 06:40

Computers and Technology, 14.01.2021 06:40



Mathematics, 14.01.2021 06:40

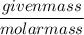
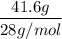 = 1.5 moles
= 1.5 moles