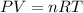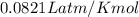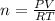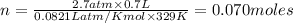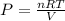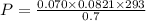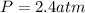Chemistry, 20.04.2020 20:18 laryans317
Nitrate salts (NO3), when heated, can produce nitrites (NO2) plus oxygen (O2). A sample of potassium nitrate is heated, and the 02 gas produced is collected in a 700 mL flask. The pressure of the gas in the flask is 2.7 atm, and the temperature is recorded to be 329 K. The value of R= 0.0821 atm L/(mol K) How many moles of O2 gas were produced? moles After a few hours, the 700 mL flask cools to a temperature of 293K. What is the new pressure due to the O2 gas?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 24.06.2019 02:00
Match the features of the organisms belonging to different kingdoms to their corresponding characteristics.
Answers: 3

Chemistry, 24.06.2019 03:30
Would entropy increase or decrease for changes in state in which the reactant is a gas or liquid and the product is a solid? what sign would the entropy change have?
Answers: 3

You know the right answer?
Nitrate salts (NO3), when heated, can produce nitrites (NO2) plus oxygen (O2). A sample of potassium...
Questions

Geography, 12.01.2020 22:31




Biology, 12.01.2020 22:31

Mathematics, 12.01.2020 22:31

Mathematics, 12.01.2020 22:31



Social Studies, 12.01.2020 22:31

Social Studies, 12.01.2020 22:31


Mathematics, 12.01.2020 22:31

Mathematics, 12.01.2020 22:31





Mathematics, 12.01.2020 22:31