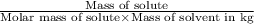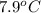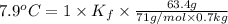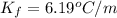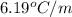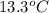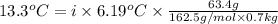Chemistry, 20.04.2020 20:50 loganrose50
When 63.4 g of glycine (C2HNO2 are dissolved in 700. g of a certain mystery liquid X, the freezing point of the solution is 7.9 °C lower than the freezing point of pure X. On the other hand, when 63.4 g of iron(III) chloride are dissolved in the same mass of X, the freezing point of the solution is 13.3 °C lower than the freezing point of pure X Calculate the van't Hoff factor for iron(III) chloride in X. Be sure your answer has a unit symbol, if necessary, and round your answer to 2 significant digits. x 10

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 21:00
The rate constant for the reaction below is 6.2 x 10−5 mol l−1 s −1. if the initial concentration of a is 0.0500 m, what is its concentration after 115 s?
Answers: 1

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
When 63.4 g of glycine (C2HNO2 are dissolved in 700. g of a certain mystery liquid X, the freezing p...
Questions


English, 08.12.2020 01:30




Mathematics, 08.12.2020 01:30




Social Studies, 08.12.2020 01:30

Mathematics, 08.12.2020 01:30

Mathematics, 08.12.2020 01:30







Mathematics, 08.12.2020 01:30

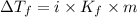
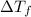 =depression in freezing point =
=depression in freezing point = 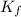 = freezing point constant
= freezing point constant