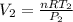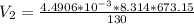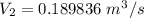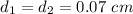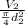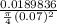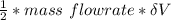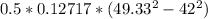Chemistry, 21.04.2020 00:03 mrashrafkotkaat
6. Air at 300°C and 130kPa ows through a horizontal 7-cm ID pipe at a velocity of 42.0m/s. (a) Calculate _ Ek W, assuming ideal-gas behavior. (b) If the air is heated to 400°C at constant pressure, what is Δ _ Ek _ Ek 400°C _ Ek 300°C? (c) Why would it be incorrect to say that the rate of transfer of heat to the gas in Part (b) must equal the rate of change of kinetic energy?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1


Chemistry, 22.06.2019 22:50
At the current rate, a graph of carbon dioxide produced by fossil fuels over time would slope upward slope downward be horizontal be vertical
Answers: 3
You know the right answer?
6. Air at 300°C and 130kPa ows through a horizontal 7-cm ID pipe at a velocity of 42.0m/s. (a) Calcu...
Questions

Mathematics, 07.07.2019 05:00

Health, 07.07.2019 05:00




History, 07.07.2019 05:00

History, 07.07.2019 05:00


History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

Social Studies, 07.07.2019 05:00



History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

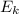

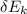 = 112.164 W
= 112.164 W 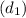 = 7 cm = 0.7 m
= 7 cm = 0.7 m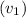 = 42 m/s
= 42 m/s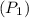 = 130 KPa
= 130 KPa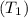 = 300°C = (300 + 273.15) = 573.15 K
= 300°C = (300 + 273.15) = 573.15 K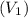 = Inlet velocity
= Inlet velocity 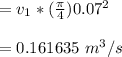
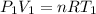
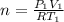
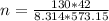
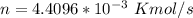
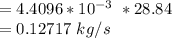
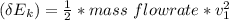
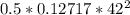
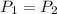 = 130 KPa
= 130 KPa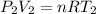
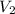 the subject of the formula; we have:
the subject of the formula; we have: