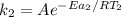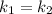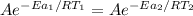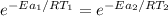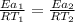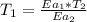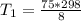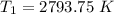Chemistry, 21.04.2020 00:11 hannah2757
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the reaction equation 2h2o2(aq)⟶2h2o(l)+o2(g) the activation energy for this reaction is 75 kj·mol−1. the enzyme catalase, found in blood, lowers the activation energy to 8.0 kj·mol−1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25 °c?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 13:00
If two objects at different te,peraure are in contact with each other what happens to their temperature
Answers: 1

Chemistry, 23.06.2019 03:30
The molar mass of nickel(ni) is 58.7 g/mol. how many moles are in an 88 gram sample of nickel?
Answers: 1

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2
You know the right answer?
Hydrogen peroxide decomposes spontaneously to yield water and oxygen gas according to the reaction e...
Questions

Advanced Placement (AP), 15.12.2020 16:20






Computers and Technology, 15.12.2020 16:20

Mathematics, 15.12.2020 16:20


History, 15.12.2020 16:20



Chemistry, 15.12.2020 16:20

Mathematics, 15.12.2020 16:20


Mathematics, 15.12.2020 16:20

English, 15.12.2020 16:20



Mathematics, 15.12.2020 16:20

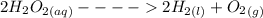
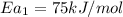
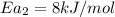
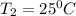 = (25+273)K = 298 K
= (25+273)K = 298 K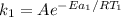 ----- equation (1)
----- equation (1)