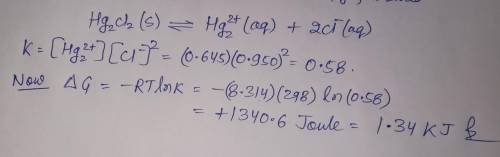Chemistry, 21.04.2020 04:51 micahwilkerson9495
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (Hg2^2+)aqueous solution, and 0.905M chloride (Cl-) aqueous solution at a temperature of 25.0°C.
Under these conditions, calculate the reaction free energy ΔG for the following chemical reaction:
Hg2Cl2(s) ⇌ Hg2^2+ (aq) + 2Cl^- (aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Asingle atom of an element has 21 neutrons, 20 electrons, and 20 protons. which element is it? ok o z
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
A chemist fills a reaction vessel with 0.623g mercurous chloride(Hg2Cl2) solid, 0.645M mercury (I) (...
Questions

English, 12.10.2019 15:50



Biology, 12.10.2019 15:50


Mathematics, 12.10.2019 15:50




Geography, 12.10.2019 15:50

Mathematics, 12.10.2019 15:50