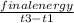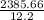Chemistry, 21.04.2020 08:47 sydneepollitt32
3. A student constructs a coffee cup calorimeter and places 50.0 mL of water into it. After a brief period of stabilization, the temperature of the water in calorimeter is determined to be 19.1 °C. To this is added 50.0 mL of water that was originally a temperature of 54.9 °C. A careful plot of the recorded temperature established T0 as 31.3 °C. What is the calorimeter constant (J/°C)?

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 17:40
Does the energy in a solid increase or decrease when changing to a liquid?
Answers: 1

Chemistry, 22.06.2019 15:30
The identities of substances are the same before and after which type of change
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 06:30
Achemist is studying the following equilibirum, which has the given equilibrium constant at a certain temperature: 2 no(g) + cl2(g) < => 2 nocl(g) kp = 2 x 10^(-6)he fills a reaction vessel at this temperature with 13. atm of nitrogen monoxide gas and 12. atm of chlorine gas. use this data to answer the questions: a. can you predict the equilibrium pressure of noci, using only the tools available to you within aleks? y/nb. if you said yes, then enter the equilibrium pressure of nocl at right. round your answer to 1 significant digit.
Answers: 1
You know the right answer?
3. A student constructs a coffee cup calorimeter and places 50.0 mL of water into it. After a brief...
Questions





Mathematics, 08.04.2020 02:14



Mathematics, 08.04.2020 02:14


Social Studies, 08.04.2020 02:14




Arts, 08.04.2020 02:14


Mathematics, 08.04.2020 02:14