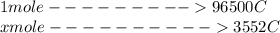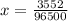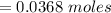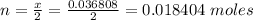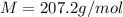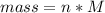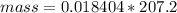Chemistry, 21.04.2020 15:30 sophcent5828
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate is reduced to lead at the cathode and oxidized to solid lead(II) oxide at the anode. Suppose a current of is fed into a car battery for seconds. Calculate the mass of lead deposited on the cathode of the battery. Round your answer to significant digits. Also, be sure your answer contains a unit symbol.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 21.06.2019 23:00
What is the maximum amount of al2(so4)3 which could be formed from 15.84 g of al and 12.89 g of cuso4?
Answers: 2

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3
You know the right answer?
Problem PageQuestion When a lead acid car battery is recharged by the alternator, it acts essentiall...
Questions



Mathematics, 07.01.2021 08:10

Computers and Technology, 07.01.2021 08:10

History, 07.01.2021 08:10

Social Studies, 07.01.2021 08:10


Mathematics, 07.01.2021 08:10

History, 07.01.2021 08:10


Mathematics, 07.01.2021 08:10

Mathematics, 07.01.2021 08:10



Mathematics, 07.01.2021 08:20

Mathematics, 07.01.2021 08:20


Mathematics, 07.01.2021 08:20

English, 07.01.2021 08:20

Mathematics, 07.01.2021 08:20

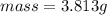
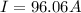
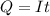
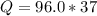
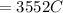
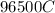
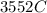 would contain how many moles
would contain how many moles