
Chemistry, 21.04.2020 16:36 enitramedouard12
Which of the following electron transitions in a hydrogen atom emits light of the shortest wavelength? Circle the correct answer and explain your answer. (No calculation needed.) A. n = 4 to n = 1 B. n = 2 to n = 1 C. n = 7 to n = 4 D. n = 4 to n = 2

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1
You know the right answer?
Which of the following electron transitions in a hydrogen atom emits light of the shortest wavelengt...
Questions








Computers and Technology, 03.12.2019 03:31

Chemistry, 03.12.2019 03:31








Mathematics, 03.12.2019 03:31


Mathematics, 03.12.2019 03:31

Health, 03.12.2019 03:31

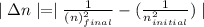
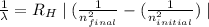
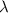 is wavelength of light emitted or absorbed,
is wavelength of light emitted or absorbed, 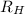 is Rydberg constant.
is Rydberg constant.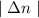 , lower will the corresponding wavelength of light.
, lower will the corresponding wavelength of light.

