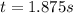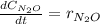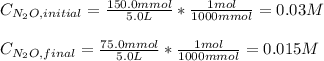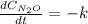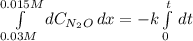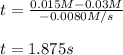Chemistry, 21.04.2020 17:30 amandajbrewerdavis
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate constant of ·0.0080Ms−1: 2N2O(g)→2N2(g)+O2(g) Suppose a 5.0L flask is charged under these conditions with 150.mmol of dinitrogen monoxide. After how much time is there only 75.0mmol left? You may assume no other reaction is important.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 03:40
In an effort to address concerns about global warming, a power plant in portland,oregon is designed to take all of its exhaust gases from its boilers and recycle the co2 using the solvay process to make sodium hydrogen carbonate. the reaction is shown below. nh3(g) + h2o(l) + co2(g) + nacl(aq) → nahco3(aq) + nh4cl(aq) how many liters each of nh3 and co2 (both at stp) would be consumed to produce 3.00 kg of sodium bicarbonate? the volume of both nh3 and co2 would be
Answers: 1

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1
You know the right answer?
Under certain conditions the rate of this reaction is zero order in dinitrogen monoxide with a rate...
Questions


Physics, 21.10.2019 13:50


Mathematics, 21.10.2019 13:50


Physics, 21.10.2019 13:50

Geography, 21.10.2019 13:50

Mathematics, 21.10.2019 13:50


History, 21.10.2019 13:50


Biology, 21.10.2019 13:50


Mathematics, 21.10.2019 13:50


English, 21.10.2019 13:50

Spanish, 21.10.2019 13:50

Mathematics, 21.10.2019 13:50

Mathematics, 21.10.2019 13:50

English, 21.10.2019 13:50