Chemistry, 21.04.2020 18:20 Isabella1319
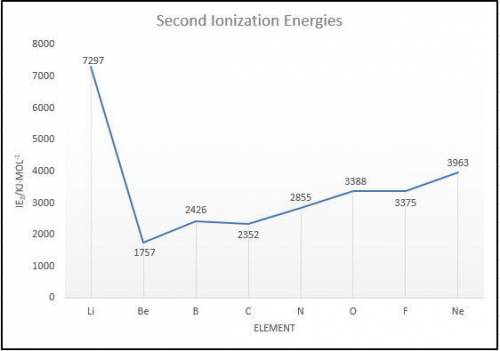
Elements in group 7A in the periodic table are called the halogens: elements in group 6A are called the chalcogens (a)
What is the most common oxidation state of the chalcogens compared to the halogens? (b) For each of the following
periodic properties. state whether the halogens or the chalcogens have larger values: atomic radii, ionic radii of the most
common oxidation state, first ionization energy. second ionization energy.

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 23.06.2019 02:30
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1
You know the right answer?
Elements in group 7A in the periodic table are called the halogens: elements in group 6A are called...
Questions

Biology, 20.04.2021 17:20



Mathematics, 20.04.2021 17:20

Mathematics, 20.04.2021 17:20

English, 20.04.2021 17:20


Mathematics, 20.04.2021 17:20


Mathematics, 20.04.2021 17:20



History, 20.04.2021 17:20