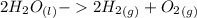Chemistry, 21.04.2020 18:47 PONBallfordM89
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseous elements. How many grams of hydrogen gas should get (theoretical yield)? The chemist collected 2.0 moles hydrogen gas. What is his percent yield?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 08:30
The characteristic of two different types of reactions are shown below. reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of and element. which statement is true about the atoms of the elements that participate in the two reactions? a: their identity changes in both reaction a and b. b: their identity changes in reaction a but not b. c: their identity changes in reaction b but not a. d: their identity remains the same.
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Using the Hoffman apparatus for electrolysis, a chemist decomposes 2.3 moles of water into its gaseo...
Questions

Social Studies, 11.02.2020 18:28




Computers and Technology, 11.02.2020 18:28

Computers and Technology, 11.02.2020 18:28





Mathematics, 11.02.2020 18:28




Mathematics, 11.02.2020 18:28

Mathematics, 11.02.2020 18:28


Mathematics, 11.02.2020 18:28

English, 11.02.2020 18:28