Chemistry, 21.04.2020 18:51 tfaulk2884
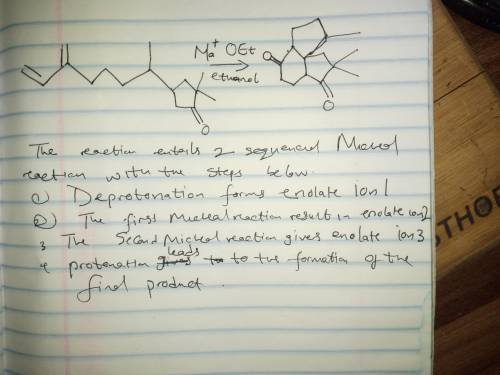
This reaction involves two successive Michael reactions, and has the following steps: 1. Deprotonation forms enolate ion 1; 2. The first Michael reaction forms enolate ion 2; 3. The second Michael reaction forms enolate ion 3; 4. Protonation leads to the final product. Write the mechanism out on a sheet of paper, and then draw the structure of enolate ion 1.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 00:10
Which of the following best describes the formation of plasma?
Answers: 1

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1
You know the right answer?
This reaction involves two successive Michael reactions, and has the following steps: 1. Deprotonati...
Questions

Mathematics, 24.10.2019 07:43

Mathematics, 24.10.2019 07:43

Social Studies, 24.10.2019 07:43

Mathematics, 24.10.2019 07:43


Mathematics, 24.10.2019 07:43




Mathematics, 24.10.2019 07:43

History, 24.10.2019 07:43

Mathematics, 24.10.2019 07:43

Biology, 24.10.2019 07:43




Spanish, 24.10.2019 07:43



Mathematics, 24.10.2019 07:43