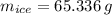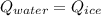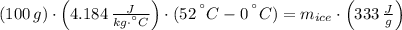Chemistry, 21.04.2020 19:22 Quanashiar
You add 100.0 g of water at 52.0 °C to 100.0 g of ice at 0.00 °C. Some of the ice melts and cools the water to 0.00 °C. When the ice and water mixture reaches thermal equilibrium at 0 °C, how much ice has melted? (The specific heat capacity of liquid water is 4.184 J/g ⋅ K. The enthalpy of fusion of ice at 0 °C is 333 J/g.) Mass of ice = g

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2
You know the right answer?
You add 100.0 g of water at 52.0 °C to 100.0 g of ice at 0.00 °C. Some of the ice melts and cools th...
Questions

Spanish, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20

French, 01.03.2021 19:20

Social Studies, 01.03.2021 19:20



History, 01.03.2021 19:20


Physics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20


Mathematics, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20


English, 01.03.2021 19:20

Mathematics, 01.03.2021 19:20