Chemistry, 21.04.2020 20:19 siriuskitwilson9408
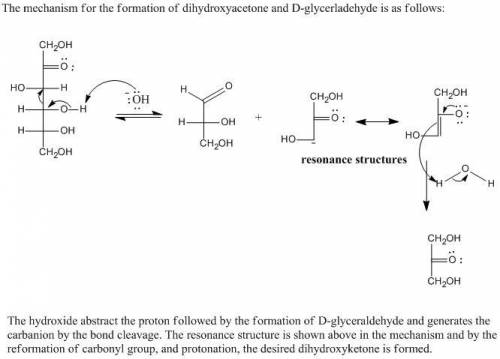
On treatment with aqueous base, D-fructose slowly undergoes cleavage to form dihydroxyacetone and D-glyceraldehyde (in low yield). The second step of this reaction produces two organic products, one of which is an anion with two resonance structures. Draw the structures of the two products formed in the second step of the reaction. Draw only one resonance structure for the anionic product.

Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1


Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
On treatment with aqueous base, D-fructose slowly undergoes cleavage to form dihydroxyacetone and D-...
Questions

Mathematics, 28.06.2019 22:00

Mathematics, 28.06.2019 22:00

Computers and Technology, 28.06.2019 22:00

Health, 28.06.2019 22:00


Mathematics, 28.06.2019 22:00



Mathematics, 28.06.2019 22:00

Mathematics, 28.06.2019 22:00

English, 28.06.2019 22:00


Mathematics, 28.06.2019 22:10

English, 28.06.2019 22:10

Biology, 28.06.2019 22:10