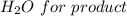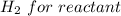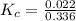Chemistry, 21.04.2020 20:39 allierl2001
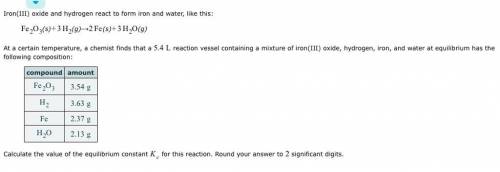
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarrow 2Fe(s) + 3H_2O(g) At a certain temperature, a chemist finds that a 5.4 L reaction vessel containing a mixture of iron(III) oxide, hydrogen, iron, and water at equilibrium has the following composition: Calculate the value of the equilibrium constant K_c for this reaction. Round your answer to 2 significant digits.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2


You know the right answer?
Iron(III) oxide and hydrogen react to form iron and water, like this: Fe_2O_3(s) + 3H_2(g) rightarro...
Questions

Mathematics, 03.06.2021 05:20


Geography, 03.06.2021 05:20



Advanced Placement (AP), 03.06.2021 05:20

Mathematics, 03.06.2021 05:20



Mathematics, 03.06.2021 05:20

Chemistry, 03.06.2021 05:20





History, 03.06.2021 05:30

Mathematics, 03.06.2021 05:30


Mathematics, 03.06.2021 05:30

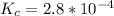
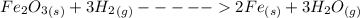
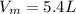
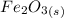 is a constant with value of
is a constant with value of 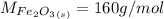
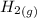 is a constant with value of
is a constant with value of 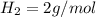
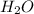 is a constant with value of
is a constant with value of 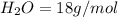
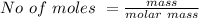
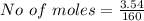
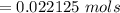
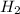
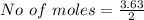
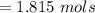
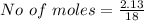
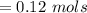
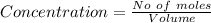
![Concentration[Fe_2 O_3] = \frac{0.222125}{5.4}](/tpl/images/0615/6667/c3dcc.png)
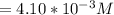
![Concentration[H_2] = \frac{1.815}{5.4}](/tpl/images/0615/6667/26a72.png)
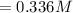
![Concentration [H_2O] = \frac{0.12}{5.4}](/tpl/images/0615/6667/14bc0.png)
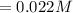
![K_c = \frac{[concentration \ of \ product]}{[concentration \ of \ reactant ]}](/tpl/images/0615/6667/26746.png)