
Chemistry, 21.04.2020 21:47 sustaitaj2022
The reaction was studied at a series of different temperatures. A plot of ln(k) vs. 1/T gave a straight line relationship with a slope of -693 and a y-intercept of -0.425. Additionally, a study of the concentration of A with respect to time showed that only a plot of ln[A] vs. time gave a straight line relationship. What is the initial rate of this reaction when [A] = 0.41 at 271 K?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Layers of rock containing fossils, like the layers illustrated here, are most likely composed of rocks.
Answers: 2

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 16:00
He table below gives the atomic mass and relative abundance values for the three isotopes of element m. relative abundance (%) atomic mass (amu) 78.99 23.9850 10.00 24.9858 11.01 25.9826 what is the average atomic mass (in amu) of element m? 2.86 5.36 24.30 24.98
Answers: 2
You know the right answer?
The reaction was studied at a series of different temperatures. A plot of ln(k) vs. 1/T gave a strai...
Questions







Chemistry, 04.11.2020 19:10


Mathematics, 04.11.2020 19:10


Mathematics, 04.11.2020 19:10


Mathematics, 04.11.2020 19:10



History, 04.11.2020 19:10

Mathematics, 04.11.2020 19:10


Physics, 04.11.2020 19:10

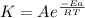
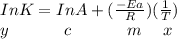
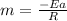
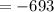
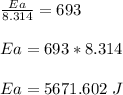
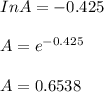
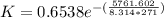
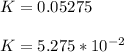
![\frac{1}{[A]}](/tpl/images/0615/8431/153b1.png) vs time is a straight line relationship;
vs time is a straight line relationship;

