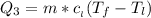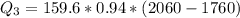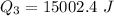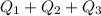Use the following information on Cr to determine the amount of heat required to convert 159.6 g of solid Cr at 1760°C into liquid Cr at 2060°C. melting point = 1860°C; boiling point = 2672°C ΔHfus = 20.5 kJ/mol; ΔHvap = 339 kJ/mol; c(solid) = 44.8 J/g°C; c(liquid) = 0.94 J/g°C Enter your answer in units of kJ to three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 21.06.2019 21:50
H2so4(aq) + mg(s)—> mgso4(aq) +h2(g) which substance is the acid in the reaction?
Answers: 3

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1
You know the right answer?
Use the following information on Cr to determine the amount of heat required to convert 159.6 g of s...
Questions

English, 21.07.2019 10:20

Mathematics, 21.07.2019 10:20



Social Studies, 21.07.2019 10:20




Mathematics, 21.07.2019 10:20

Health, 21.07.2019 10:20


Mathematics, 21.07.2019 10:20

Health, 21.07.2019 10:20

Mathematics, 21.07.2019 10:20


Mathematics, 21.07.2019 10:20

Biology, 21.07.2019 10:20

Mathematics, 21.07.2019 10:20


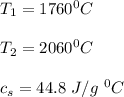
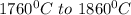 is calculated as:
is calculated as: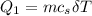
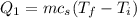
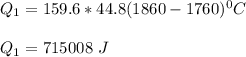
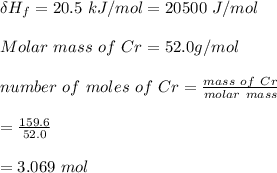
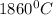 is;
is;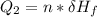
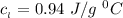
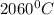 is;
is;