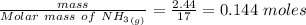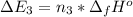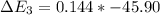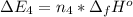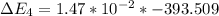Chemistry, 21.04.2020 22:23 asdf334asdf334
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 296.84 kJ/mol) Enthalpy change = kJ 0.22 mol of decomposes to and ( for = –90.83 kJ/mol) Enthalpy change = kJ 2.44 g of is formed from and excess ( for = –45.90 kJ/mol) Enthalpy change = kJ mol of carbon is oxidized to ( for = –393.509 kJ/mol) Enthalpy change = kJ

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
What are the charges of the subatomic particles by choosing the answer from the drop down menu. protons have a (+1,0,or-1). (protons,neutrons,electrons) have a 0 charge. 3.) electrons have a (+1,0,-1)
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Calculate enthalpy changes for the following: 0.047 g of sulfur (rhombic) burns, forming ( for = – 2...
Questions






Mathematics, 29.03.2021 03:30


Mathematics, 29.03.2021 03:30





Mathematics, 29.03.2021 03:30


Mathematics, 29.03.2021 03:30


Mathematics, 29.03.2021 03:30



Mathematics, 29.03.2021 03:30

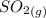 (
( 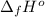 for
for 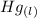 and
and 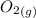 (
( 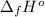 for
for 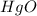 = –90.83
= –90.83 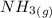 is formed from and excess
is formed from and excess 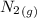 and excess
and excess 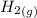 (
( 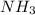 = –45.90 kJ/mol)
= –45.90 kJ/mol) 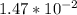 mol of carbon is oxidized to
mol of carbon is oxidized to 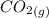 (
( 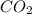 = –393.509 kJ/mol)
= –393.509 kJ/mol) 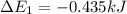
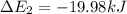
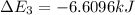
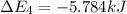
 ) =
) = 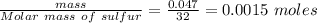
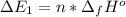
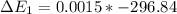
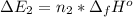
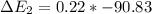
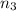 ) =
) =