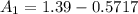Chemistry, 21.04.2020 22:35 tiniecisneros28
A concentration cell based on the following half reaction at 289 K has initial concentrations of 1.39 M , 0.312 M , and a potential of 0.037206 V at these conditions. After 9.1 hours, the new potential of the cell is found to be 0.0095376 V. What is the concentration of at the cathode at this new potential

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1
You know the right answer?
A concentration cell based on the following half reaction at 289 K has initial concentrations of 1.3...
Questions

English, 03.02.2021 21:20




Social Studies, 03.02.2021 21:20


Chemistry, 03.02.2021 21:20


Biology, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20


Biology, 03.02.2021 21:20

Mathematics, 03.02.2021 21:20


Chemistry, 03.02.2021 21:20


Mathematics, 03.02.2021 21:20


Spanish, 03.02.2021 21:20

Social Studies, 03.02.2021 21:20

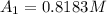
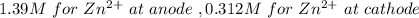
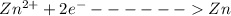
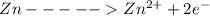
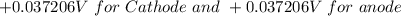
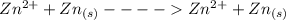
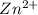 at cathode loss some concentration and
at cathode loss some concentration and 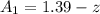
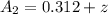
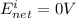
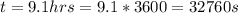
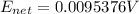
![E_{cell} = E^i_{cell}- \frac{RT}{nF} ln[\frac{[A_2]}{[A_2]} ]](/tpl/images/0616/0641/f278f.png)
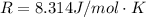
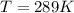
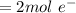
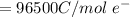
![0.0095376 =0.0 -\frac{[8.314][288]}{[2] [9600]} ln [\frac{(0.312 +z)}{(1.39 -z)} ]](/tpl/images/0616/0641/c9c95.png)
![0.0095376 = 0.12406383 \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/96214.png)
![\frac{0.0095376 }{ 0.12406383} = \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/52f0e.png)
![0.0768766 = \ ln [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/3085d.png)
![e^{0.0768766 }= [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/42989.png)
![1.0799 = [\frac{( 0.312 + z)}{[1.39 -z]} ]](/tpl/images/0616/0641/39ed8.png)
![[1.39 -z ]1.0799 = 0.312 + z \\\\1.501 - 1.0799z = 0.312 +z\\\\1.501-0.312 = 1.0799z + z\\\\1.1891 =2.0799z\\\\ z =\frac{1.1891}{2.0799}\\\\ z =0.5717](/tpl/images/0616/0641/109ca.png)