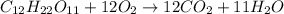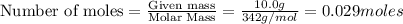Chemistry, 21.04.2020 23:31 Sillydork4545
The equation represents the combustion of sucrose. C12H22O11 + 12O2 Right arrow. 12CO2 + 11H2O If there are 10.0 g of sucrose and 8.0 g of oxygen, how many moles of sucrose are available for this reaction? 0.029 mol 0.250 mol 0.351 mol 3.00 mol

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3



Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1
You know the right answer?
The equation represents the combustion of sucrose. C12H22O11 + 12O2 Right arrow. 12CO2 + 11H2O If th...
Questions

Geography, 23.11.2020 20:10


Mathematics, 23.11.2020 20:10

Spanish, 23.11.2020 20:10

Geography, 23.11.2020 20:10

World Languages, 23.11.2020 20:10


Geography, 23.11.2020 20:10


English, 23.11.2020 20:10

Mathematics, 23.11.2020 20:10

Chemistry, 23.11.2020 20:10


Mathematics, 23.11.2020 20:10

Mathematics, 23.11.2020 20:10

History, 23.11.2020 20:10

History, 23.11.2020 20:10



 of particles.
of particles.