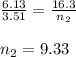Chemistry, 22.04.2020 00:10 Affousietta
A flexible container at an initial volume of 6.13 L contains 3.51 mol of gas. More gas is then added to the container until it reaches a final volume of 16.3 L. Assuming the pressure and temperature of the gas remain constant, calculate the number of moles of gas added to the container. number of moles of gas: mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 22.06.2019 20:30
Which of the following is not true about the atomic model of substances?
Answers: 1

Chemistry, 23.06.2019 00:50
Which of the following warnings would an agricultural chemist tell a farmer who wants to recycle his or her own ammonia? recycling ammonia is a difficult process that sometimes takes weeks. recycling ammonia requires a degree in biochemistry or a related field. recycling ammonia can be harmful because it is highly flammable and toxic. recycling ammonia costs too much money considering the price of the necessary chemicals.
Answers: 1

Chemistry, 23.06.2019 02:00
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
A flexible container at an initial volume of 6.13 L contains 3.51 mol of gas. More gas is then added...
Questions


Mathematics, 17.06.2020 22:57

Mathematics, 17.06.2020 22:57


Mathematics, 17.06.2020 22:57

Physics, 17.06.2020 22:57



History, 17.06.2020 22:57


Mathematics, 17.06.2020 22:57



Computers and Technology, 17.06.2020 22:57

Computers and Technology, 17.06.2020 22:57


History, 17.06.2020 22:57



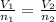
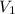 and
and 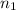 are the initial volume and number of moles
are the initial volume and number of moles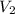 and
and 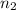 are the final volume and number of moles
are the final volume and number of moles